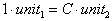 ,
,
In the physical sciences, and life in general, the
numerical part of a measurement is meaningless if there are no
units reported with it. If you tell someone you just ran 26, they
have no idea if you mean meters, miles, or something else. It
is for this reason that all physical measurements in this course
and all your other courses need to include units as part of the
answer. Dimensional analysis, or the balancing of units, is one
of the easiest ways to do a fast check of mathematical calculations
because if the units in the final answer don't make sense, then
the numerical part is also probably incorrect. For example, if
you are calculating the density of a saline solution and finish
with units of cm/sec, you have made a big mistake somewhere. Dimensions
are the physical nature of a quantity (e.g. mass, length, time,
etc.), are treated just as algebraic quantities, and can, and
should, be carried through all equations. You can only add or
subtract physical quantities if their dimensions (and preferably
their units of measure, but more on this in a moment) are the
same. Just like you cannot further simplify apples + oranges,
you cannot add or subtract two quantities with different dimensions,
say length and mass.
Your text has a brief discussion of dimensional analysis in Appendix B under the heading of unit conversion. Unit conversion is very important because you are often given a measurement in a units that are not those you need to work the problem. In this case you must convert the units so you can properly carry the dimensions through the problem, and/or you may need to report your answer in units other than those with which you finish the calculation. There are examples of this aspect of dimensional analysis on pages A11-A12 of your text, and this point will not be expounded on further here other than to indicate unit conversions are of the form
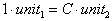 ,
,where C is a constant. This allows you to multiply or divide parts of equations by factors of the form
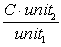
since they are equal to one.
There is more to dimensional analysis, however, than
simple unit conversion. Many units are combinations of dimensions
and their respective units. For example, the SI unit of measure
for force is the Newton (N) and is defined as (kgms-2).
There are other derived units of measure that you will regularly
encounter, and knowing the basic units that comprise them will
greatly help the solving of problems. Knowing the proper units
and dimensions can also help you recall the ordering of some basic
formulas by simply making sure the units cancel properly.
A sample problem is included at the end of this handout, solved
with three different, but equivalent, systems of notation, just
so you are familiar with each.
With all that in mind, I would just like to conclude
with a word of advice. The largest problem students have with
this course and other similar courses is that they only develop
a passive understanding of the material. Most students
have little problem understanding the basic concepts presented
in some general form. The difficulty comes at test time for one
reason or another--sometimes it's just an off day, or you run
out of time, or something else. Most commonly, however, the problem
is the lack of an active understanding of the material.
An active understanding involves using the material in new situations
not identical to the example or homework problems. All too often
you hear students say "Oh, that's easy," after someone
explains a test problem to them. This is the classic sign of a
passive understanding of the material. Homework problems are the
key to overcoming this problem, and the more problems you work,
the more easily you will be able to adapt the material to new
situations. To this end, if you are experiencing difficulty, it
will be useful to work more problems than just those assigned
As an aid the answers to all odd numbered problems are in the
back of your text. The importance of this cannot be over stressed,
but ultimately you just need to find what works best for you.
Example:
A certain object has 10.0 cal of kinetic (translational) energy and is moving at a rate of 3.00x104 cm/s. What is the mass of this object in grams?
Solution:
The formula with which to solve this problem is
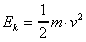 ,
,
where Ek is the translational energy in Joules,
m is the mass in kilograms, and v is the velocity
in m/s. Since m is the unknown, the first step is
to rearrange the equation:
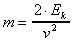 .
.
Now simple substitution and canceling of units will give the desired
result. There are three general ways to keep track of the units
in this equation:
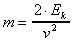
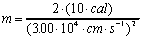
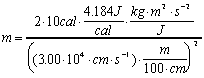

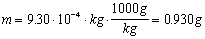
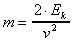
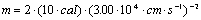
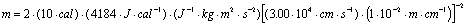
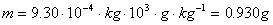
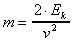
Note the conversion of Joules to its basic units
using the relation J=kgm2s-2 and
the other three simple unit conversions. Also note the use of
significant figures.
Funding supported by the Camille and Henry Dreyfus Foundation special grant program in the chemical sciences and by a subcontract under NSF grant number DUE-9455972.